Many epithelial tissues (e.g., in our lungs or gut) need to defend themselves constantly against environmental stresses (e.g. from pathogens, chemical or mechanical insults). When these epithelial cells are damaged or lost, they are often replenished by stem cells. Thus, an epithelium’s ability to sense stress and stimulate stem cell proliferation is vital to maintain its integrity and prevent tissue dysfunction, degeneration, inflammation and disease.
Therefore, a better understanding of epithelial maintenance and regeneration will help develop therapies for tissue regeneration, inflammatory diseases and cancer.
Our goal is to determine how stress signalling evokes fast and adaptive cellular responses that promote epithelial renewal and regeneration.
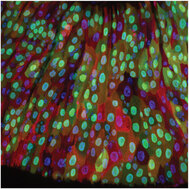
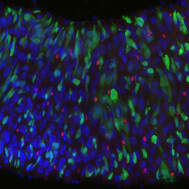
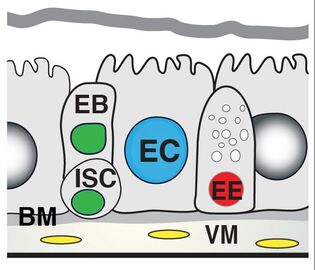
To this aim, we are using the adult Drosophila (fruit fly) intestine (midgut), which is an outstanding model to study homeostatic turnover, regeneration, early tumorigenesis and ageing in stem cell-based epithelia. Like our own intestine, the fly intestine is maintained by intestinal stem cells (ISCs) that divide to produce progenitors such as enteroblasts (EBs) that differentiate into nutrient-absorptive enterocytes (ECs) and enteroendocrine precursors (EEPs) that differentiate into hormone-producing enteroendocrine cells (EEs). Fly and mammalian ISCs also use many of the same molecular cues to promote their survival, proliferation and differentiation.
The adult fly intestine enables the use of sophisticated genetic tools to trace ISC lineages, perform rapid loss- and gain-of-function studies (and screens) in a cell type-specific manner and independently manipulate multiple cell types at the same time. In addition, its simple tissue architecture allows for easy imaging and quantitative analysis of the entire organ. Furthermore, we can identify and FACS isolate each cell type using specific markers and determine their transcriptome using RNA sequencing. Altogether, these tools allow us to perform incisive in vivo experiments that cannot be currently performed in other models.
Using the adult Drosophila intestine and its unparalleled genetics, combined with proteomics, transcriptomics, and fixed or live imaging, we will determine how stress signalling promotes epithelial maintenance and regeneration focussing on the three research questions below.
Therefore, a better understanding of epithelial maintenance and regeneration will help develop therapies for tissue regeneration, inflammatory diseases and cancer.
Our goal is to determine how stress signalling evokes fast and adaptive cellular responses that promote epithelial renewal and regeneration.
To this aim, we are using the adult Drosophila (fruit fly) intestine (midgut), which is an outstanding model to study homeostatic turnover, regeneration, early tumorigenesis and ageing in stem cell-based epithelia. Like our own intestine, the fly intestine is maintained by intestinal stem cells (ISCs) that divide to produce progenitors such as enteroblasts (EBs) that differentiate into nutrient-absorptive enterocytes (ECs) and enteroendocrine precursors (EEPs) that differentiate into hormone-producing enteroendocrine cells (EEs). Fly and mammalian ISCs also use many of the same molecular cues to promote their survival, proliferation and differentiation.
The adult fly intestine enables the use of sophisticated genetic tools to trace ISC lineages, perform rapid loss- and gain-of-function studies (and screens) in a cell type-specific manner and independently manipulate multiple cell types at the same time. In addition, its simple tissue architecture allows for easy imaging and quantitative analysis of the entire organ. Furthermore, we can identify and FACS isolate each cell type using specific markers and determine their transcriptome using RNA sequencing. Altogether, these tools allow us to perform incisive in vivo experiments that cannot be currently performed in other models.
Using the adult Drosophila intestine and its unparalleled genetics, combined with proteomics, transcriptomics, and fixed or live imaging, we will determine how stress signalling promotes epithelial maintenance and regeneration focussing on the three research questions below.
ROS and Epithelial RegenerationUpon damage to the fly intestinal epithelium, intestinal epithelial cells produce reactive oxygen species (ROS) and activate stress signalling that promote regeneration (Patel et al., 2019). Using loss- and gain-of-function genetics, proteomics and transcriptomics, we are determining how ROS promote intestinal regeneration by identifying ROS-controlled regulators of regeneration and by determining the molecular responses to ROS and stress signalling.
|
Circadian Control of Epithelial MaintenanceMany tissues are rhythmically exposed to environmental stress, due to circadian organismal behaviour. Using fixed and live-imaging, we are investigating whether circadian-activated cues regulate intestinal epithelial homeostasis.
|
Regulation of Epithelial Cell LifespanWhen new fly intestinal epithelial cell (IECs) production by ISCs is inhibited, IECs increase their lifespan to prolong epithelial maintenance (Jin, Patel et al., 2017). Using transcriptomics and loss- and gain-of-function genetics, we are determining adaptive mechanisms that extend IEC lifespan when new cell production is blocked.
|